The Rise of Software as a Medical Device in Healthcare Innovation
The healthcare industry has experienced an unprecedented transformation over the last decade, driven by advancements in technology. One of the most revolutionary developments has been the rise of Software as a Medical Device (SaMD). This innovative concept is reshaping how healthcare providers diagnose, monitor, and treat patients, without the need for traditional hardware-based devices. As digital health continues to evolve, SaMD is quickly becoming a cornerstone in modern medical practices. But what exactly does this mean for the future of healthcare? And how are software development company is contributing to this shift? Let’s explore the rise of SaMD and its role in healthcare innovation.
What is Software as a Medical Device (SaMD)?
Software as a Medical Device (SaMD) refers to any software intended to be used for medical purposes without being part of a physical device. In simple terms, SaMD is a software application that performs functions that traditionally required a hardware device—such as diagnostic, therapeutic, or monitoring tasks.
For example, mobile apps that monitor heart rate or software that analyzes medical imaging data are considered SaMD. Unlike traditional medical devices, which rely on hardware to perform their functions, SaMD relies purely on software, making it a highly flexible and scalable solution.
The Role of SaMD in Healthcare Innovation
SaMD is at the forefront of digital health innovation, a rapidly expanding field that leverages technology to improve healthcare outcomes. The increasing reliance on SaMD reflects a broader trend of digital transformation within healthcare. Let’s dive deeper into some of the key benefits and innovations driven by SaMD:
1. Enhanced Patient Monitoring and Management
SaMD has revolutionized patient monitoring by enabling real-time tracking of patient data through wearable devices, mobile apps, and cloud-based solutions. This continuous flow of information allows healthcare providers to make more informed decisions and intervene proactively. According to a report by Global Market Insights, the wearable medical device market, which includes SaMD applications, is expected to surpass $60 billion by 2027, reflecting its growing impact on patient care.
For instance, remote patient monitoring (RPM) software allows physicians to track chronic conditions like diabetes and hypertension remotely, reducing the need for frequent hospital visits. These tools improve the efficiency of healthcare delivery, lower costs, and enhance patient convenience.
2. Personalized Healthcare and AI Integration
Artificial intelligence (AI) and machine learning are playing a crucial role in SaMD’s ability to offer personalized care. AI-driven SaMD applications are capable of analyzing vast amounts of patient data to identify patterns and make predictions about disease progression or treatment effectiveness. This capability not only helps in diagnosing diseases earlier but also tailors treatment plans to the individual patient.
In 2023, FDA-approved AI-based SaMD solutions like IDx-DR, an AI-powered diagnostic tool for diabetic retinopathy, have showcased how software can offer accurate diagnostic support, leading to faster interventions and improved patient outcomes.
3. Improved Diagnostics and Decision Support
SaMD applications are increasingly being used for diagnostic purposes. These tools aid healthcare professionals by providing decision support, improving diagnostic accuracy, and reducing human error. For example, AI-based medical imaging software can detect signs of cancer, heart disease, and other critical conditions from radiological images far quicker than traditional methods.
AI-powered diagnostic tools are particularly beneficial in areas with a shortage of specialized medical professionals. In such cases, SaMD can act as a valuable supplement, ensuring that no patient is left undiagnosed or untreated.
4. Regulatory Considerations and Compliance
As SaMD continues to evolve, so do the regulatory frameworks surround it. The FDA and the European Medicines Agency (EMA) have been working to develop guidelines that ensure SaMD products are safe and effective for use. In 2021, the FDA released new guidelines for SaMD, recognizing that software applications can be just as critical to patient care as traditional medical devices.
The regulatory environment ensures that SaMD maintains high standards of safety and efficacy, which in turn boosts trust and adoption within the healthcare industry.
The Growing Role of Software Development Companies
As the demand for digital health innovation grows, software development companies play a pivotal role in the creation and deployment of SaMD applications. These companies are tasked with developing cutting-edge software that meets rigorous regulatory standards while offering tangible benefits to healthcare providers and patients.
Software development companies are focusing on areas such as:
- Integration with IoT Devices: Connecting medical software with IoT-enabled devices is becoming increasingly important. IoT connectivity allows for seamless data collection and sharing, which is essential for real-time decision-making.
- Data Security: With patient data being at the core of SaMD applications, software developers are prioritizing data security to comply with regulations like HIPAA and GDPR.
- Cloud Computing: Cloud-based SaMD platforms provide healthcare professionals with easy access to patient data, enabling more efficient collaboration and faster decision-making.
- User Experience (UX): Healthcare professionals and patients alike need user-friendly interfaces that allow them to interact with complex medical data easily. Developers are focusing heavily on improving the UX to ensure that SaMD tools are intuitive and effective.
Key Stats on SaMD Adoption and Market Growth
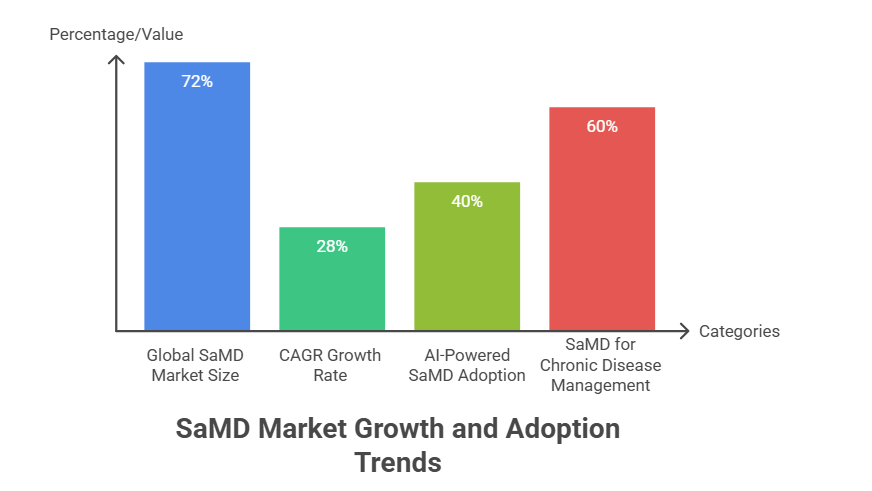
- The global SaMD market size was valued at $72 billion in 2022 and is expected to grow at a CAGR of 28% from 2023 to 2030. This rapid growth is driven by the increasing demand for remote healthcare solutions, as well as advancements in AI and cloud computing.
- 40% of healthcare organizations have adopted AI-powered SaMD applications for diagnostics, patient management, and treatment planning, reflecting the increasing trust in digital tools.
- Over 60% of healthcare providers are now using SaMD for chronic disease management, with many more planning to integrate these solutions within the next few years.
Challenges and Future Outlook
Despite its tremendous potential, the rise of SaMD is not without challenges. These include:
Data Privacy and Security: As SaMD applications handle sensitive patient data, ensuring that this information remains secure is a top priority. The industry must continue to evolve its data protection mechanisms to prevent breaches.
Interoperability: SaMD solutions need to seamlessly integrate with existing healthcare systems, such as Electronic Health Records (EHR), to provide comprehensive patient care.
Regulatory Uncertainty: As technology evolves rapidly, regulatory bodies are continuously updating guidelines for SaMD. The challenge is to balance innovation with the need for safety and efficacy.
Final Thoughts
The rise of Software as a Medical Device (SaMD) is undoubtedly one of the most exciting developments in healthcare innovation today. As SaMD continues to evolve, the integration of AI, machine learning, and wearable technologies will drive even greater efficiency, cost-effectiveness, and patient outcomes. Software development companies are central to this transformation, playing a key role in the creation and scaling of SaMD solutions that are not only safe and effective but also revolutionary in their ability to improve patient care.
At App Maisters, we specialize in developing cutting-edge SaMD solutions that meet the highest regulatory standards while pushing the boundaries of what digital health innovation can achieve. As the healthcare industry continues to embrace digital transformation, we are proud to be at the forefront of this exciting journey, helping healthcare providers deliver better care to their patients through innovative software solutions.
FAQs
What is Software as a Medical Device (SaMD) and how does App Maisters contribute?
SaMD is software used for medical purposes, like monitoring or diagnosis, without the need for physical devices. App Maisters develops cutting-edge SaMD solutions that help healthcare providers deliver more efficient, personalized care.
How does App Maisters use AI in Software as a Medical Device?
At App Maisters, we integrate AI into SaMD to improve data analysis, enabling quicker diagnoses, more accurate predictions, and personalized patient care. This leads to better health outcomes and smarter healthcare solutions.
What benefits does SaMD bring to healthcare providers, and how does App Maisters help?
SaMD helps healthcare providers with real-time monitoring, early disease detection, and personalized care. App Maisters designs SaMD applications that enhance healthcare delivery by making it more efficient and accessible.
How does App Maisters ensure SaMD is regulated and compliant?
App Maisters ensures all our SaMD solutions comply with industry standards set by regulatory bodies like the FDA and EMA, guaranteeing both safety and effectiveness in healthcare applications.
Can SaMD improve patient care, and how does App Maisters make that happen?
Yes, SaMD improves patient care by enabling real-time monitoring and early intervention. App Maisters creates SaMD solutions that help healthcare providers offer personalized, timely care for better patient outcomes.
Why should healthcare providers trust App Maisters with SaMD development?
Healthcare providers can trust App Maisters for SaMD development because we specialize in building reliable, compliant, and innovative medical software that enhances patient care and boosts operational efficiency.
What role does App Maisters play in the future of SaMD?
App Maisters is dedicated to driving the future of SaMD by creating innovative solutions that help healthcare providers deliver high-quality, patient-centered care, while staying ahead of regulatory requirements.




















